100 doctors set up Immuno-Oncological Society to frame treatment norms
An innovation by IIT-Bombay may help reduce the cost of a type of immunotherapy used to treat blood cancer to a tenth of that in the US.
“We filed for a patent last month for the manufacture of the CAR-T cell therapy and hope it would eventually be available at a tenth of the US cost,” said Dr Rahul Purwar from IITB’s biosciences department. It would take another couple of years before the medicines are available for clinical trial at Tata Memorial Hospital, Parel.
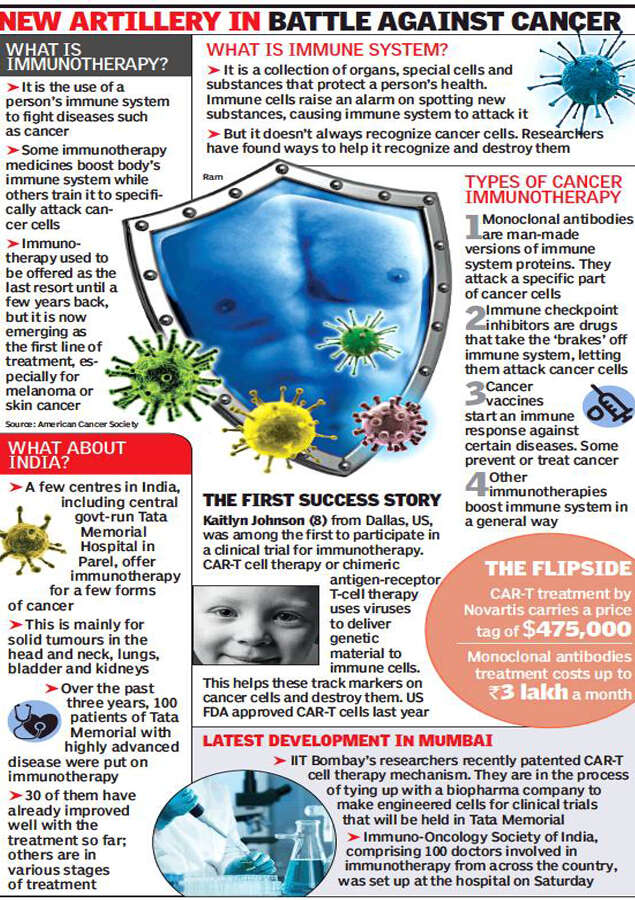
CAR-T cell therapy is one of the many immunotherapy agents that promise to treat cancer by tweaking a patient’s own immune system. While immunotherapy agents were used as the “last resort” for patients with advanced cancer till a few years back, it is emerging as the chosen treatment for certain cancers such as melanoma or that of the skin.
At a scientific meeting held at Tata Memorial on Saturday, around 100 doctors from across the country who are interested in immunotherapy, set up Immuno-Oncological Society of India. “This has been done to popularize and set up guidelines for this youngest branch of cancer treatment in India,” said medical oncologist Dr Jyoti Bajpai.
Earlier, cancer treatment was about surgery, chemotherapy and radiation. “But the nascent branch of immunotherapy is recording developments in cancer treatment on a monthly or weekly basis,” said her colleague Dr Gaurav Narula.
In the three years since Tata Memorial started a type of immunotherapy, 100 patients with advanced or metastatic form of the disease have been using it. “Around 30 patients are doing well,” said Dr Kumar Prabhash, adding it is too early to assess others on treatment.
The only hitch is the cost; the 100 patients at Tata Memorial pay from their pocket or insurance for the Rs 2-3 lakh a month treatment. One of their metastatic lung cancer patients has been on this treatment for 25 months and “feeling much better”, said Dr Prabash.
The IIT-B patent would cut the cost of only one type of immunotherapy that helps blood cancer patients. “We will tie up with a pharmaceutical company for manufacture and should be ready for clinical trial within a year or two,” said Purwar from IIT. The trial will be held at Tata Memorial. CAR-T cell therapy requires drawing blood from patients and separating out their T cells. A disarmed virus is then used to genetically change the T cells to produce chimeric antigen receptors or CARs. When injected, CARs targets blood cancer cells.
Source:https://health.economictimes.indiatimes.com/news/medical-devices/100-doctors-set-up-immuno-oncological-society-to-frame-treatment-norms/63063628
“We filed for a patent last month for the manufacture of the CAR-T cell therapy and hope it would eventually be available at a tenth of the US cost,” said Dr Rahul Purwar from IITB’s biosciences department. It would take another couple of years before the medicines are available for clinical trial at Tata Memorial Hospital, Parel.

CAR-T cell therapy is one of the many immunotherapy agents that promise to treat cancer by tweaking a patient’s own immune system. While immunotherapy agents were used as the “last resort” for patients with advanced cancer till a few years back, it is emerging as the chosen treatment for certain cancers such as melanoma or that of the skin.
At a scientific meeting held at Tata Memorial on Saturday, around 100 doctors from across the country who are interested in immunotherapy, set up Immuno-Oncological Society of India. “This has been done to popularize and set up guidelines for this youngest branch of cancer treatment in India,” said medical oncologist Dr Jyoti Bajpai.
Earlier, cancer treatment was about surgery, chemotherapy and radiation. “But the nascent branch of immunotherapy is recording developments in cancer treatment on a monthly or weekly basis,” said her colleague Dr Gaurav Narula.
In the three years since Tata Memorial started a type of immunotherapy, 100 patients with advanced or metastatic form of the disease have been using it. “Around 30 patients are doing well,” said Dr Kumar Prabhash, adding it is too early to assess others on treatment.
The only hitch is the cost; the 100 patients at Tata Memorial pay from their pocket or insurance for the Rs 2-3 lakh a month treatment. One of their metastatic lung cancer patients has been on this treatment for 25 months and “feeling much better”, said Dr Prabash.
The IIT-B patent would cut the cost of only one type of immunotherapy that helps blood cancer patients. “We will tie up with a pharmaceutical company for manufacture and should be ready for clinical trial within a year or two,” said Purwar from IIT. The trial will be held at Tata Memorial. CAR-T cell therapy requires drawing blood from patients and separating out their T cells. A disarmed virus is then used to genetically change the T cells to produce chimeric antigen receptors or CARs. When injected, CARs targets blood cancer cells.
Source:https://health.economictimes.indiatimes.com/news/medical-devices/100-doctors-set-up-immuno-oncological-society-to-frame-treatment-norms/63063628
Comments
Post a Comment